Resource • Article
Disease Management: Finding an Epidemiological Foothold for COVID-19 Clinical Trials


Updates related to COVID-19 are now an everyday occurrence as the coronavirus continues its spread on a global scale. Additionally, existing coronavirus information remains dynamic and changeable:
- Timelines originally placed the start of the pandemic in December 2019 in Wuhan, China. However, sources now note that patient zero may have been infected over four months earlier.
- Basic symptoms of the disease have been expanded per the CDC, but the coronavirus continues to surprise the scientific community with new presentations and comorbidities.
- International disease epicenters are shifting, with the United States as the focal point for new cases as of the writing of this article.
“The truth is that COVID-19 is brand new, completely unique,” notes Mark Vieder, VP of Drug Safety and Pharmacovigilance at Biorasi. “While it does share some similar pathology with other infectious and pulmonary diseases, COVID-19 is defined by its multiple unknowns and that makes executing clinical trials challenging.”
Visualizing the COVID-19 Trial Landscape
Pharmaceutical companies and the scientific community are stepping into the ever-changing COVID-19 landscape – swiftly and carefully – in order to develop vaccines and treatment for the coronavirus. For vaccines, there are currently more than 180+ trials in the pre-clinical stage as of July 2020. Only two have been approved for limited, emergency use. See Figure 1 below for further details:
Figure 1: Current COVID-19 Trials
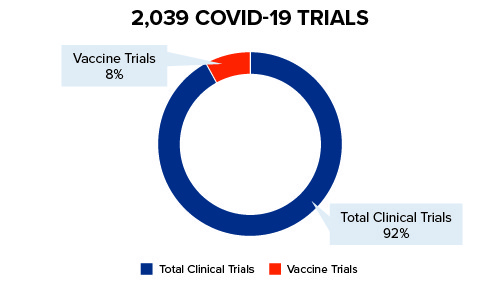

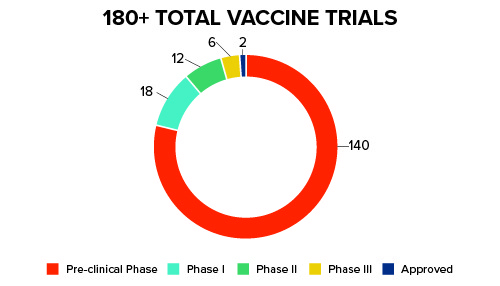

Trial Resources in the Time of COVID-19
“Researchers currently investigating potential vaccines and treatments for COVID-19 infections have had to absorb a lot of information quickly prior to committing to trial feasibility,” says Vieder.
Key among the information that must be analyzed by companies investing time and resources into COVID-19 vaccines and treatment is the ever-shifting epidemiology:
- While some similarities exist between COVID-19 (also known as SARS-CoV-2), the 2003 SARS virus (SARS-CoV), and influenza strains from 1918 and 2009, COVID-19 is a unique disease.
- COVID-19 is more infectious than its disease counterparts and harder to contain.
- A majority of patients infected with COVID-19 can be asymptomatic or present mild symptoms.
- COVID-19 patients that present symptoms are five to six times more at risk of hospital admission to ICUs than the 2009 flu.
- Disease mortality affects a more elderly population (70+ years of age), unlike the 1918 and 2009 flu strains, and includes more comorbidities.
Overall, this data presents a conflict between the disease ecosystem and patient demographic: while the current landscape offers greater access to a large number COVID-19 infected patients at a hospital or clinic site, resources for clinical trials are more constrained as countries focus on managing the public health crisis associated with the coronavirus rather than researching its transmission and infection.
Additionally, the window for developing vaccines and moving the product to market will likely become smaller as more vaccines become approved and made available.
Embracing Regulatory Requirements
In addition to the shifting nature of the coronavirus and infected patient demographics, regulatory updates – such as those provided by the FDA, EMA, Health Canada, and other international regulatory agencies – require quick review and response, often mid-trial. For example, in addition to providing guidance for conducting clinical trials safely and efficiently during the pandemic, the FDA has also created the Coronavirus Treatment Acceleration Program (CTAP).
Through CTAP, the FDA provides the following:
- Status updates on existing trials and treatment types – ranging from antivirals to antibodies
- References for developing COVID-19-related treatments including:
- How to engage with the FDA and expediting clinical trials
- General advice on developing vaccines and biological treatments
- Emergency use authorization and treatment guidelines
Moving Faster (and Smarter) than the Disease
Preparing for a COVID-19 trial may be a novel experience for many drug developers. Companies willing to take the risk of developing, testing, and marketing coronavirus vaccines must be able to move faster than the disease, be flexible enough to pivot and adapt when necessary, and be smart enough to leverage past clinical trial expertise and best practices in guiding the trial to completion.
To move forward toward execution, it is imperative to optimize existing trial expertise to defuse potential challenges. These may include:
- Global Experience – To finish trials quickly and optimize access to the patient demographic required, expanding trials internationally is a robust strategy. Executing clinical trials on a global scale comes with its own set of requirements, however. Companies will need to rely on their past experience in international clinical trials and recognize how compliance, confidentiality, and other regulations differ and require additional time or approval to begin the clinical trial.
- Regulatory Experience – International regulatory agencies are focused on finding answers to COVID-19 epidemiology. It is important to engage with the FDA and similar agencies as new guidelines and disease information becomes available. While the spirit of competition is one of the drivers for vaccine research, following the progress of industry peers via the CTAP dashboard may provide both a snapshot overview and detailed updates on what to expect at all stages of coronavirus studies – however, please note that the CTAP pathway may not fit all COVID-19 trials/protocols.
- Clinical Trial Experience – Understanding COVID-19 and the differences (and similarities) of previous pandemics is paramount. Companies that have led clinical trials across infectious diseases and pulmonary/respiratory therapeutic indications will have an advantage in applying lessons learned to COVID-19 studies. Leveraging a knowledge base of best practices from related trials adds the necessary agility for these studies, as well as providing a proactive strategy in recognizing potential delays and obstacles. Strong communication with clinical sites and relationships with disease experts can also provide the support needed during these trials.
- Decentralization Experience – Decentralization is another area that can be optimized for COVID-19 trials. Decentralized trials represent a spectrum of options designed to ensure patient safety and efficiency. While most COVID-19 trials will be site based due to the disease criteria and demographic needs, remote solutions can remove challenges to recruiting by expanding trials on a global scale, add oversight to patient and site staff, enforce safety compliance measures, and enable stopped studies to move forward in virtual environments.
Understanding COVID-19 is only one part of the execution strategy. Our next article will focus on the importance of site selection and patient safety during coronavirus studies. Click here to learn more in the Biorasi COVID-19 Article Series.
References:
- Corum, Jonathan, et al. “Coronavirus Vaccine Tracker.” New York Times, August 3, 2020.
- Petersen, Eskild, M.D., et al. “Comparing SARS-CoV-2 with SARS-CoV and Influenza Pandemics.” The Lancet, July 3, 2020.
- Seladi-Schulman, Jill, Ph.D. “COVID-19 vs. SARS: How Do They Differ?” Healthline, April 2, 2020.
- Mphahlele, Jeffrey. “COVID-19 Vaccine: the Challenges of Running a Trial in the Middle of a Pandemic.” The Conversation, July 6, 2020.